We serve over 40 diverse labs allowing us to produce a variety of instruments including those used for:
Animal Behavior
Animal behavior provides a non-invasive measure of neural computation. After all, reacting to stimuli is what nervous systems evolved to do. We have designed equipment to stimulate and record behavior from a variety of organisms.

Dowling Lab: Projected images of drifting gratings are used to drive the visual optokinetic response of larval zebrafish. The response of multiple fish (6-12) can be recorded and analyzed in real time.
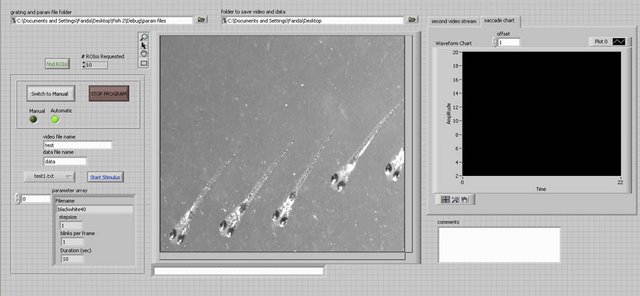
Dowling Lab: Software used to generate visual stimulus, record and analyze eye movements.
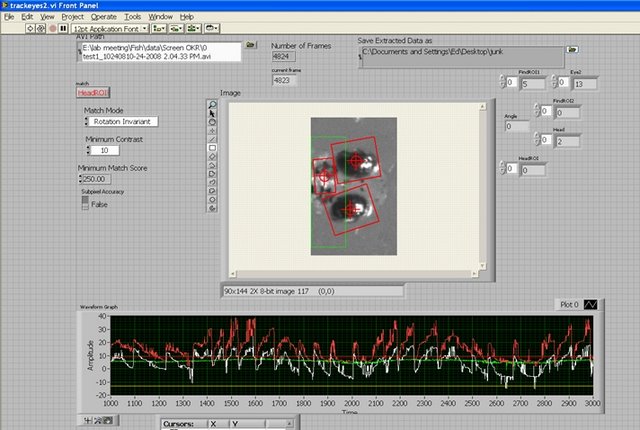
Dowling Lab: Software running off-line provides an automatic and precise measurement of eye position, temporal registered to the visual stimulus.
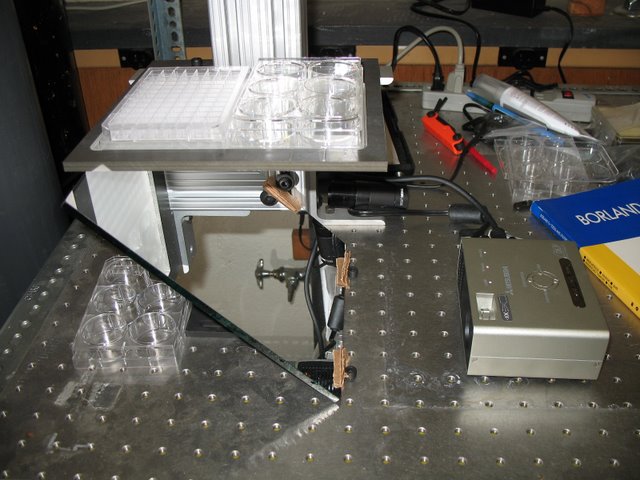
Dowling Lab: Equipment designed to simultaneous monitor the swimming behavior of up to 180 fish in response to varying illumination conditions.

Olveczky Lab: Working with Olveczky lab members, custom isolation chambers were designed and constructed to segregate conditioning cages. Water ports can be seen along the back of the chrome rack.
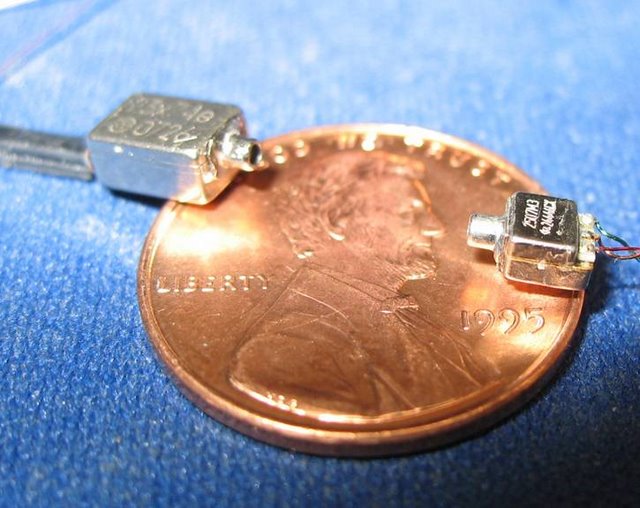
Olveczky Lab: By mounting a tiny microphone on the head of a zebra finch, it is possible to minimize variations in song recordings that arise from changes in the position and orientation of the bird. A miniature speaker directly driving the bird’s auditory system can alter what the animal hears without affecting acoustic recordings.
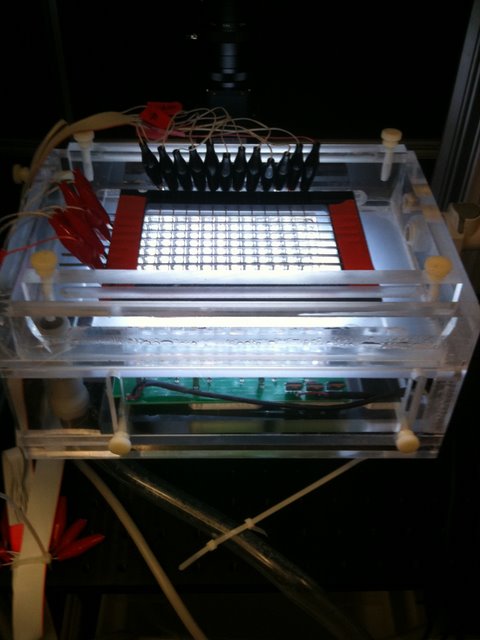
Schier Lab: The chamber shown allows machine vision software to provide an addressable, controlled, mild, electrical stimulus to each of 96 wells containing a larval zebra fish. By tickling the fish in this manner, the Schier lab can explore the effects of altered sleep patterns on behavior.

Uchida Lab: A rodent behavioral chamber designed to measure the subject’s value of immediate and delayed reward. Variable numbers of food pellets can be delivered to each food window, access to which is under temporal control. Retractable levers report the animal’s response.

Uchida Lab: Two of many computer-controlled 16 channel olfactometers capable of producing precise mixtures of odors and variable concentrations. They are part of a larger system of hardware and software produce by the Neuroengineering facility designed to measure the behavior of rodents in a nose-poke olfactory discrimination task.
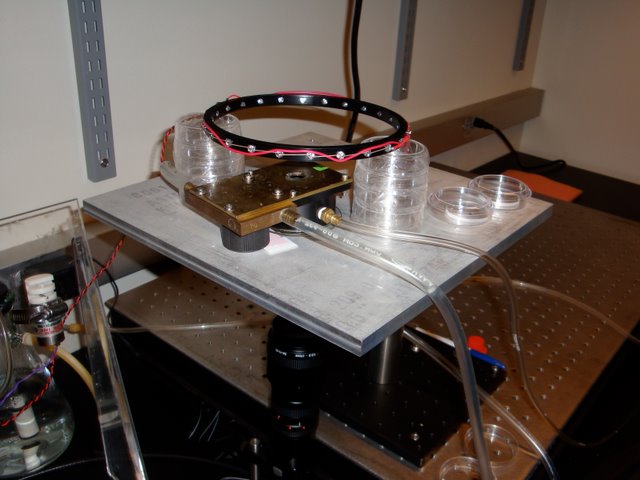
Zhang Lab: Equipment for measuring the turning behavior of C. elegans in the presence of odorants. The leftmost hose maintains stage temperature while the right hose delivers odor laden air to a chamber that houses the worm suspended in a hanging water droplet. Responses are recorded by the camera underneath.

Zhang Lab: Worm tracking “microscope”. Machine vision software maintains the camera above a target-worm within a 14” x 14” arena. Other custom software analyzes body motion, speed, turn probability, etc.
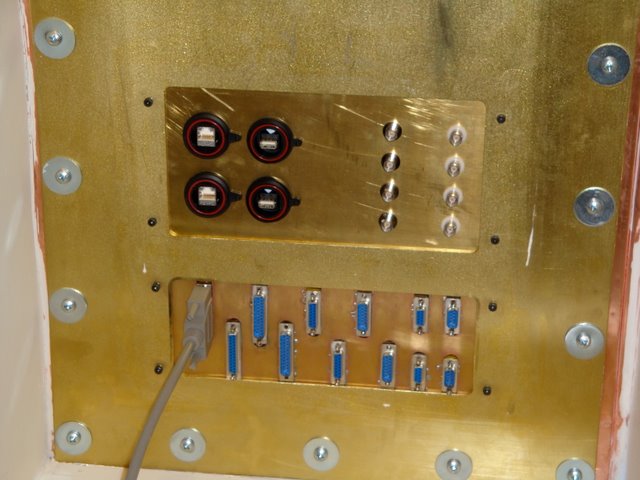
Buckner Lab/MRI facility: Patch panel maintains shielding while allowing cabling to reach into the scan area.
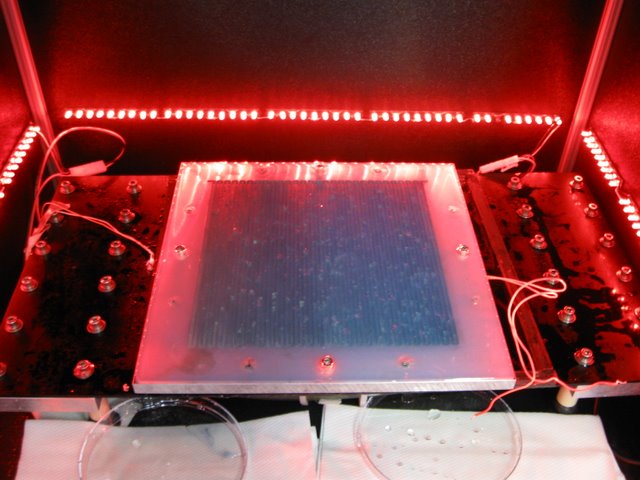
Samuel Lab: An actual experiment tracking the response of multiple worms in the presence of a gradient. Apparent surface imperfections are simply beads of condensed water that result from the humidity of the chamber.













Electron Microscopy
Much of the anatomical structure of the nervous system is too small to be resolved with an optical microscope. For this reason, CBS has taken on an ambitious scientific effort known as the "Connectome Project". We have developed high speed imaging hardware as well as software allowing the visualization of large amounts of data.

Reid Lab: T.E.M.C.A., may be the worlds fastest electron microscope, capable of continuously generating images at rates as high as 15 Mpixels/s. Many times faster than commercially available systems, it was fabricated for a fraction of the price. The Neurotechnology facility played a role in nearly every stage of this project.

Reid Lab: Unlike a traditional T.E.M. in which an image is formed at the level of the film chamber (white rectangular flanked by squares), a large vacuum chamber allows the electron image to expand, much like the magnified image created by a movie projector. Steel and lead shielding is removed for photo.

Reid Lab: A large phosphor screen at the end of the path is imaged by four overlapping 11 Mpixel CCD cameras.
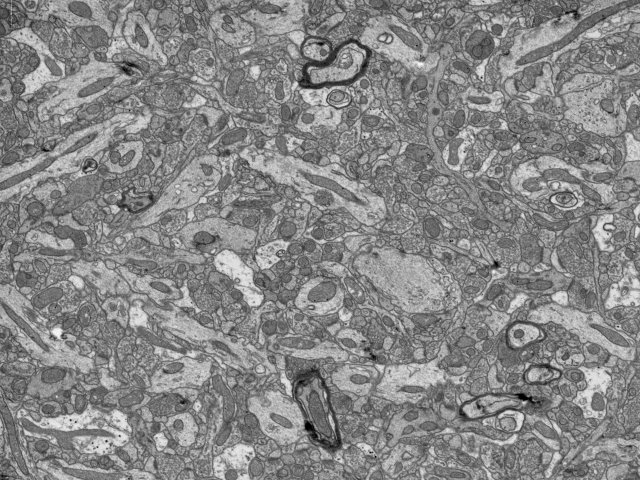
Reid Lab: An early image taken from the microscope.

Lichtman Lab: Scanning electron microscopes will play a large role in fulfilling the goals of the Connectome Project. We have provided software and hardware support; occasionally assisting the manufacturers with custom installations.
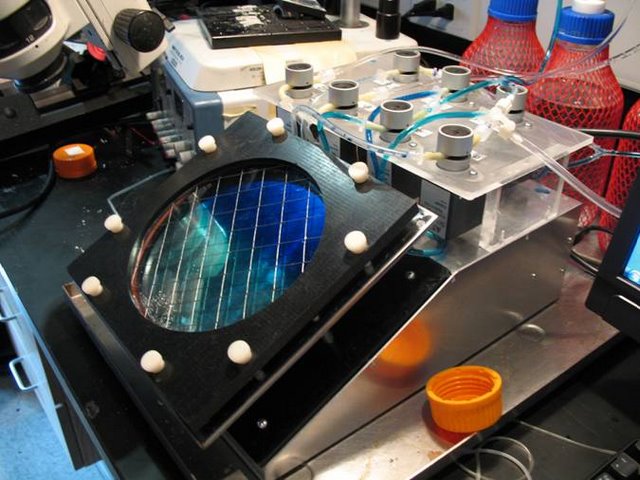
Lichtman Lab: In order to reproducibly stain large volumes of tissue sectioned by the A.T.L.U.M., we designed and fabricated this fully automated device. It provides temperature controlled staining with up to two solutions and a rinse. During prototyping, solution exchange was monitored with dyes.
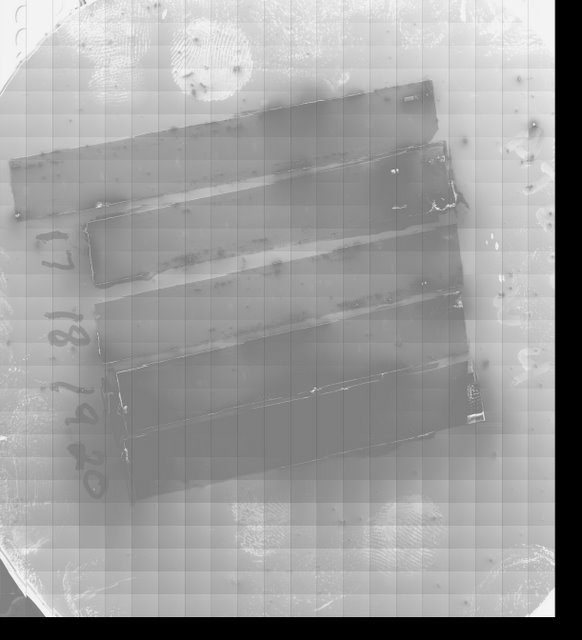
Lichtman Lab: The goals of the Connectome Project often demand features that were not implemented by microscope manufacturers. Shown here is an image of a four inch silicon wafer holding five strips of A.T.L.U.M. tape. In order to attain such a low power image, we created a custom microscope interface and image acquisition system.
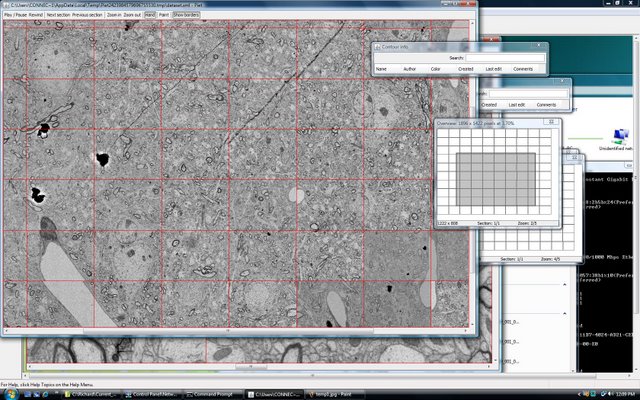
Lichtman Lab: Working with Duncan Mak, we created software capable of visualizing nearly boundless amounts of data. While many excellent commercial programs exist (Photoshop, Image J, etc.) most can not handle the 100’s of Gigabytes of data that are required by the Connectome. Red lines delineate individual overlapping 20Mpixel images that are part of a much larger sheet.
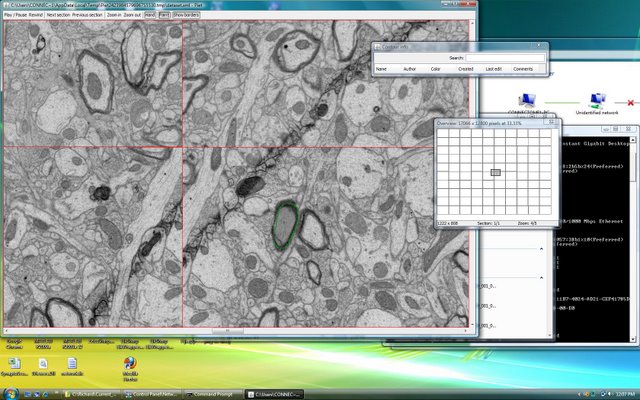
Lichtman Lab: The same data shown at higher magnification. The software also allows for searchable markup (green contour). We have tested its performance with image sheets as large as 1.5 Terabytes








Optical Microscopy
Optical microscopy plays a central role in neuroscience beyond the profound contributions of Neuroanatomy. Visualizing living neural tissue allows for guided electrophysiology or the measure of neural function using dyes, genetic probes, or intrinsic responses.

Meister Lab: This Olympus microscope has been modified to allow for patch clamp recordings from retinal ganglion cells. The stage has been replaced with a stable fixed table onto which the preparation and manipulator are mounted. Translating the F.O.V. is accomplished by moving the microscope.
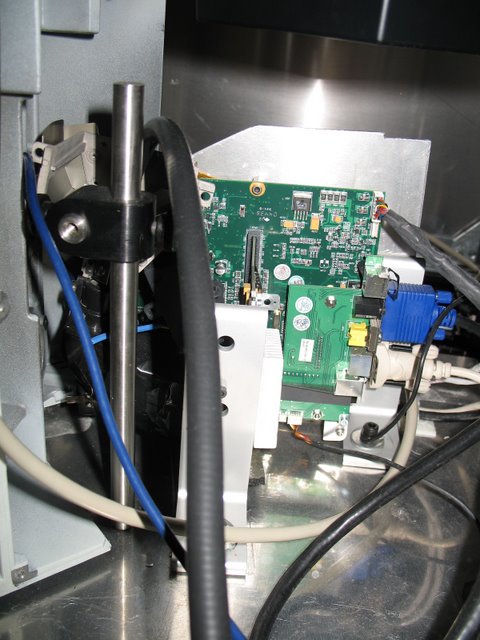
Meister Lab: The same microscope from behind. The trans-illumination lamp has been removed and replaced with a digital projector. This allows patterned images to be presented to the retain while cellular responses are recorded. Incompatible E.M.F. sources have been removed or modified.

Lichtman Lab: Similar modifications were made to this Nikon microscope to allow for patch and extracellular recordings. Custom Faraday cage, fixed stage, and microscope translator are visible.
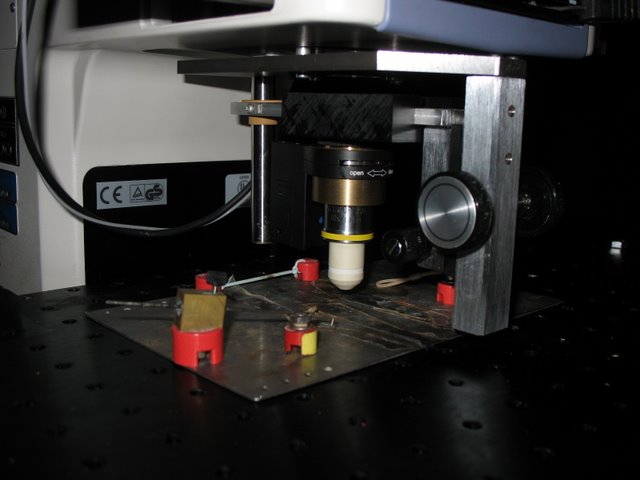
Lichtman Lab: Custom objective changing/focusing unit was added to account for the Nikon’s lack of a turret focus.
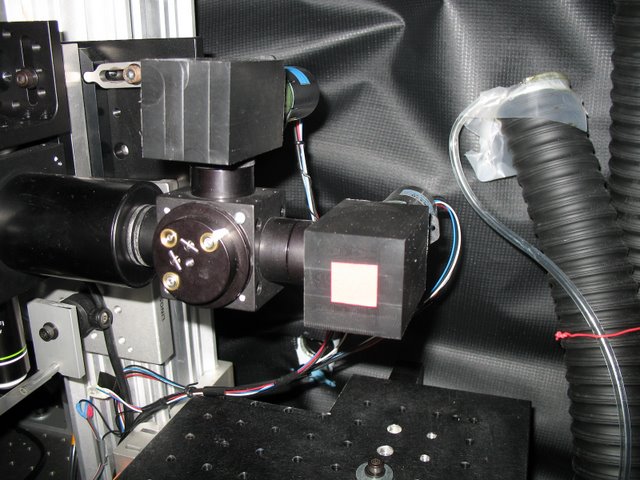
Murthy Lab: We have produce numerous devices for the many custom built 2 photon microscopes that exist in CBS. Here two PMT mounts are shown as part of a two channel, two photon microscope.

Reid, Wilson, Engert Labs: GaAs detectors used in 2 photon microscopy are very sensitive and easily damaged by excess light. These custom shutters are part of a protection system. They are designed to be very thin so as not to reduce the amount of light arriving at the detector. The central aperture is 1” in diameter.

Wilson Lab: A variation of the shutter design, integrated into a beam splitting cube.

Meister/Murthy Labs: A dual fiber, two channel L.E.D. light source capable of rapidly switching between N.I.R. and 465 nm illumination. By synchronizing illumination with video acquisition it was possible to simultaneously measure olfactory neural responses via intrinsic and synaptophluorin signals.

Murthy Lab: A mount and driver electronics for a 5W blue Phlat light L.E.D. manufactured by Luminus. Lower power devices have been constructed for the Uchida, Murthy, Engert, and Meister labs.

Meister Lab: A custom designed and fabricated photolithography station. This tool allows the Meister lab to fabricate their 61 channel planar electrodes arrays

Murthy/Uchida Labs: Adaptors to convert an extracellular electrode manipulator into a nanoliter injection system used for virus delivery.

Landisman Lab: A slice patch clamp rig with integrated microscope. This microscope was designed to deliver D.I.C.-like image quality for visually guided patch recording. It provides a very stable and open platform and cost one-sixth the price of a traditional scope.

Landisman Lab: Living neurons imaged using our patch microscope. Expensive D.I.C. optics are replaced with shallow angle N.I.R. illumination.













Electrophysiology
Electrophysiological tools give us a direct measure of neural function. We have extensive experience recording from neurons intra/extra-cellularly. We have designed a variety of amplifiers, signal conditioners, data acquisition software and data analysis programs.
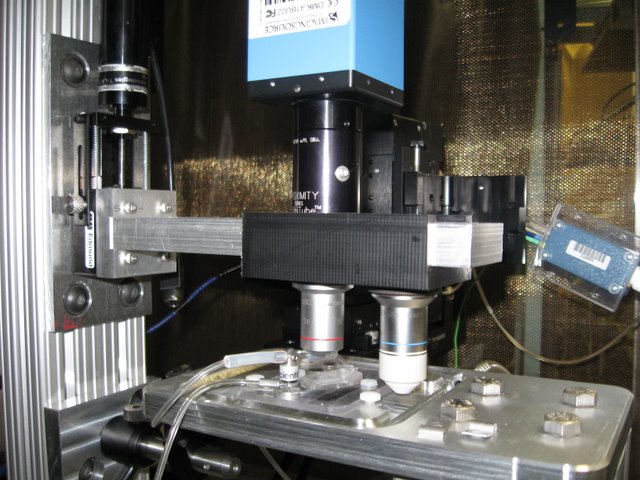
Landisman Lab: Sliding objective mount and computer controlled focus allow for simplified magnification changes when recording electrodes are in place.

Meister Lab: Tobi Sutz is using one of a collection of programs created to record and visualize 64 channels of electrophysiological data. This particular program works with a wired version of the Litke device.

Meister/Dulac/Kreiman Labs: Intan Technology produces a commercially available 16 channel amplifier that functions similar to the Litke chip. We are helping to integrate this chip into research equipment for multiple labs. The P.C.B. shown was made by Joe Bergan of the Dulac lab.
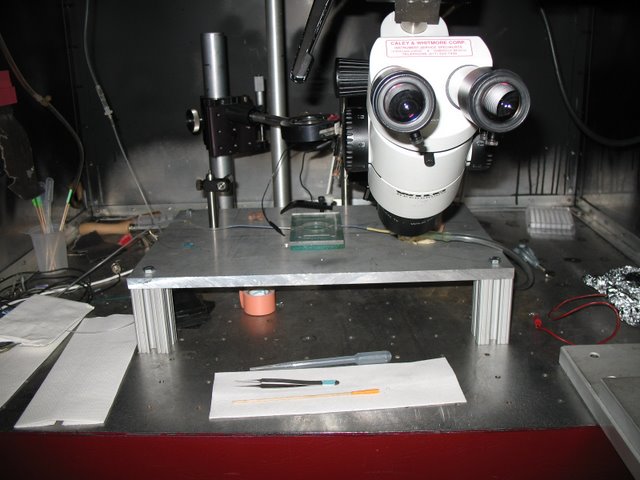
Dowling Lab: A simple rig used to record extracellularly from larval zebra fish retina.

Meister Lab: A custom open frame stereotatic device.

Murthy/Uchida labs: Precision titanium head plates in a variety of shapes and sizes, each optimized for particular experimental constraints.

Engert Lab: Head stage adaptors required for extended reach and clearance.

Schier Lab: Driver for a rapid-response temperature controlled microscope stage instert. The unit is capable of changing temperature at a rate of 2 degrees C/s.
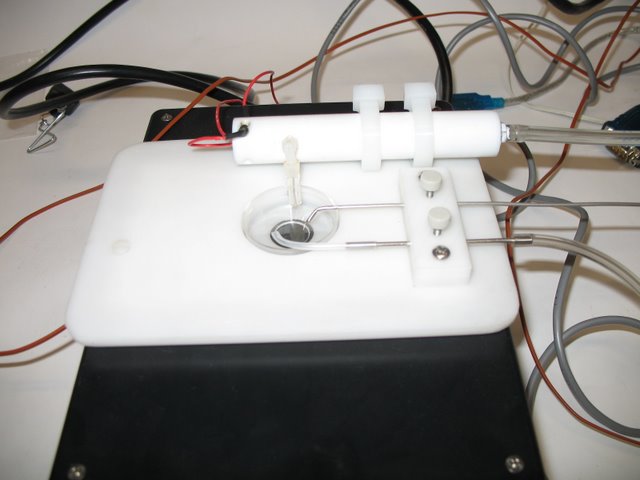
Schier Lab: Temperature controlled micrsocpe stage instert. Perfusion inflow, outlet and tempearture probes are visible. Insert accomadates standard plastic dishes.








